Faller Packaging: Reliable serialisation and aggregation of folding cartons
Who Is Faller Packaging?
Faller Packaging, a leading provider of pharmaceutical packaging solutions, places the highest priority on process reliability when it comes to the serialization of folding cartons. To ensure that serial numbers on Bollini labels are accurately captured, verified, and reported back, an advanced inspection system from QualiVision AG has been implemented.
Serialization plays a crucial role in the pharmaceutical supply chain by ensuring traceability, preventing counterfeiting, and complying with international regulatory standards such as the EU Falsified Medicines Directive (FMD). By integrating a high-performance vision system into its production line, Faller Packaging not only reinforces product safety but also strengthens its commitment to innovation, quality assurance, and regulatory compliance.
“With the inspection system from QualiVision, we have gained a reliable and future-proof solution for the aggregation and traceability of Bollini labels. The seamless integration, high detection accuracy, and ease of use are impressive in daily operation. We particularly value the complete aggregation, precise label inspection, and the flexibility in sampling and labelling. The collaboration was professional, efficient, and characterised by a genuine understanding of our requirements.”
– Faller Packaging Project Team, 2025
Customer Perspective – Enhancing Efficiency and Ensuring Quality
To meet the increasing complexity and growing regulatory requirements in pharmaceutical packaging, we at Faller Packaging have implemented a state-of-the-art camera system from QualiVision in our production. Our goal was to significantly improve the positioning and inspection of applied self-adhesive labels, while also ensuring the secure aggregation of Bollini labels – a key requirement for the Italian market.
Key Customers Benefits:
Higher packaging reliability through precise and automated label control
Improved product safety thanks to full serial number traceability
Reduced manual rework, lowering the risk of human error
Full compliance with national and international pharmaceutical regulations
Scalable system that can adapt to future requirements and market needs
Faster, more efficient production processes with consistent quality assurance

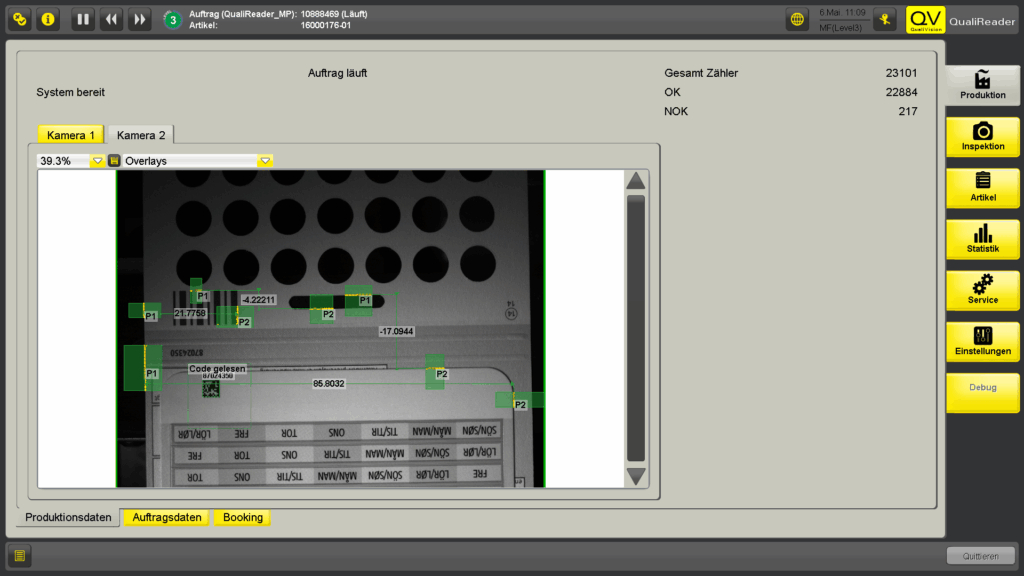
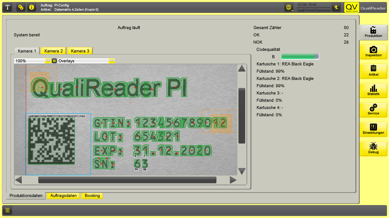
The system enables automated detection and verification of label placement, checks for complete application, and records serial numbers with full traceability. Faulty units are reliably detected and rejected, resulting in a significant reduction in manual rework.
Since its implementation, we have benefited from a much more efficient quality assurance process. Automated inspection ensures that all serialisation and traceability requirements are consistently met – a critical contribution to product safety and regulatory compliance.
The system is modular in design and can be flexibly expanded as needed – for example, to meet new country-specific requirements or to accommodate additional label variants. This makes it not only a solution to current challenges, but also a key element in the future-proof development of our production lines.

Highlights of the solution:
- 100% Serial Number Tracking: Each number is uniquely recorded and assigned the status OK / NOK / QA
- Inspection and Measurement of Label Position: For maximum precision
- Automatic Rejection of Defective Products: With complete documentation
- Direct Integration with Customer System: Data provision at the end of production, runtime query also possible
- Reliable Aggregation at Carton Level: With sandwich labels customizable via layout tool
- Flexible Handling of Random Samples: Manual marking, removal, and tracking
- User-Friendly HMI: For easy operation of all components
- Reliable Continuous Operation (24/7): Up to 500 products per minute
- Automated Report Generation: For quality assurance and traceability
- Setup Mode: With separate recording and automatic removal of test numbers