Manual vs Automated Visual Inspection
in Pharma, Medtech & Food Packaging
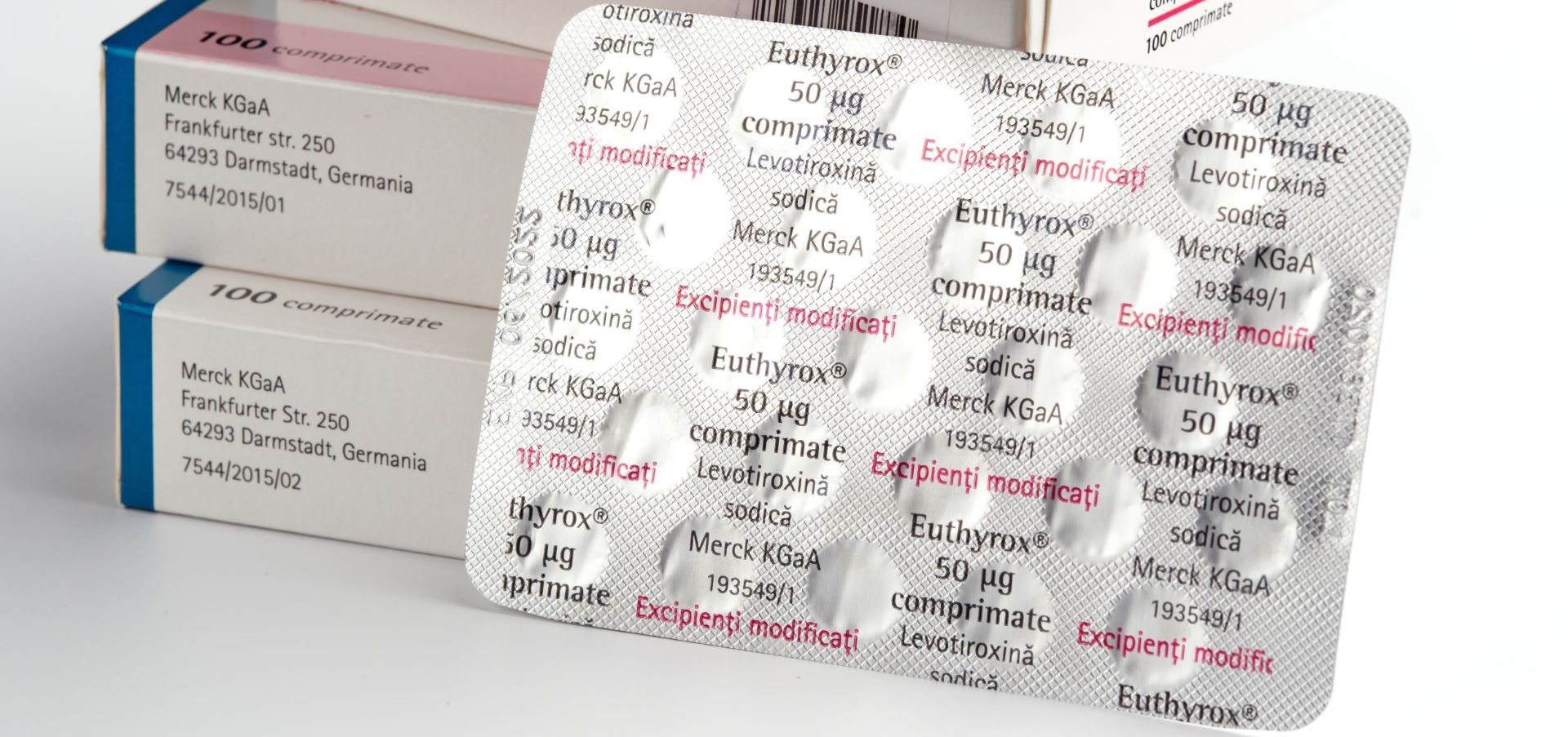
Visual inspection plays a vital role in quality assurance across pharmaceutical, medtech, and food manufacturing environments. It ensures that products are free from visible defects such as particles, cracks, incorrect fill levels, or contamination — using either manual or automated systems.
Manual Visual Inspection (MVI) is typically used for small batches or as a backup step, while Automated Visual Inspection (AVI) is better suited for high-volume, standardized production lines. Each method offers specific advantages and limitations regarding efficiency, consistency,
What Is Manual Visual Inspection (MVI)?

Manual Visual Inspection relies on trained human operators to examine products for visible defects such as foreign particles, surface damage, or improper labeling. This method is often used in low-volume production, clinical trial batches, or where flexibility and human judgement are essential.
While MVI offers a relatively low-cost and adaptable solution, it is prone to subjectivity and fatigue. Consistency between operators can vary, and documentation is typically limited unless supported by additional systems.
What Is Automated Visual Inspection (AVI)?

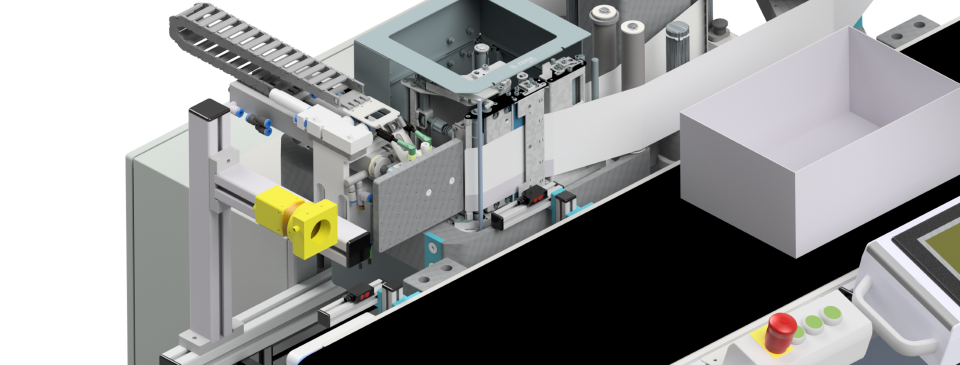
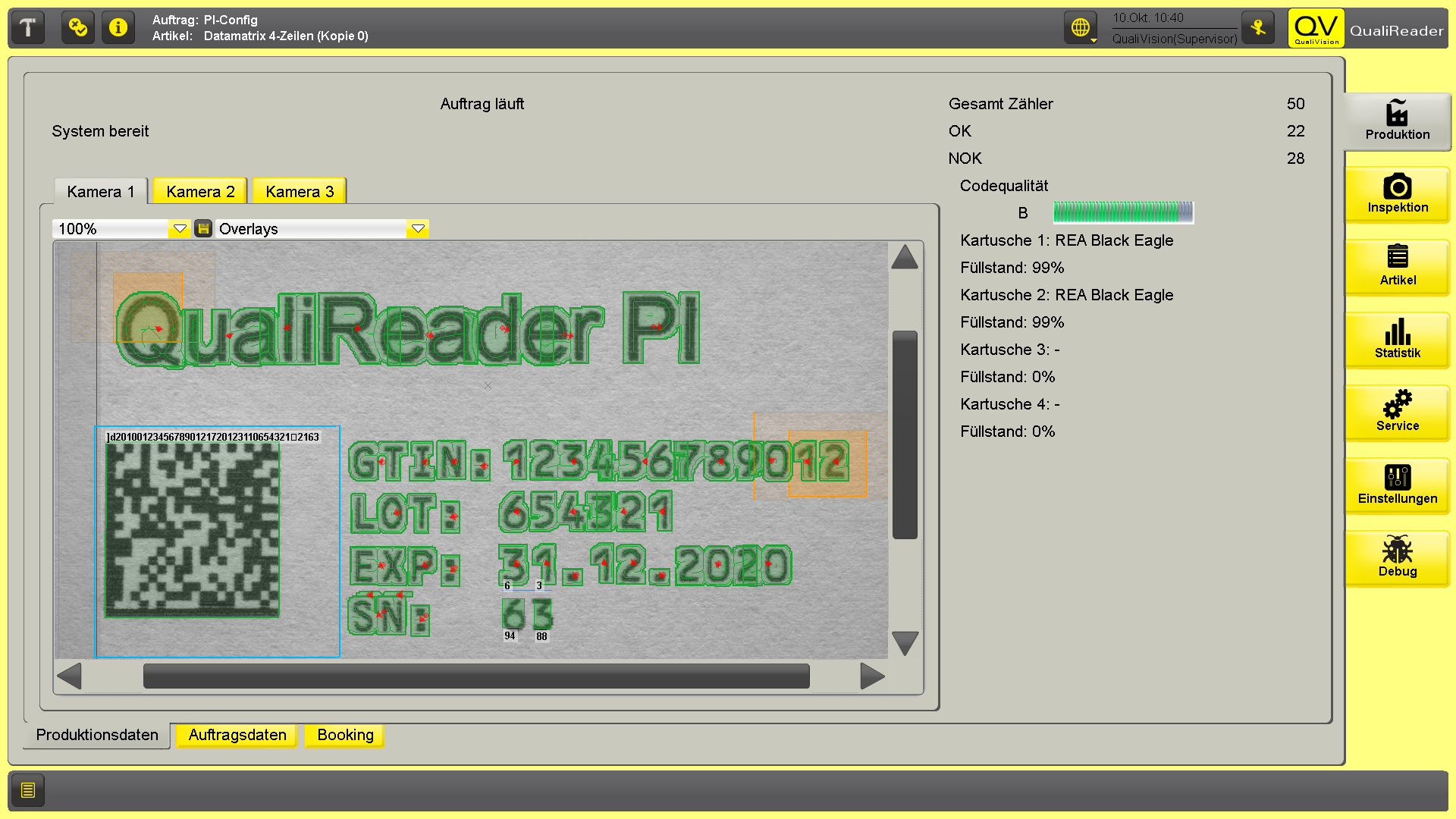
Automated Visual Inspection uses high-resolution cameras, lighting systems, and advanced software (often powered by AI or machine learning) to inspect products in-line during production. AVI systems can detect even subtle defects in real time and provide comprehensive documentation, including image storage and audit trails.
These systems are highly efficient, consistent, and scalable, making them ideal for large-scale manufacturing in regulated industries like pharmaceuticals and medtech. However, initial setup costs and technical complexity are significantly higher compared to manual inspection.
Comparison of Manual and Automated Visual Inspection in Pharma, Medtech and Food Production
This comparison highlights the key advantages and disadvantages of Manual Visual Inspection (MVI) and Automated Visual Inspection (AVI). Both are widely used across regulated industries such as pharmaceuticals, medical devices, and food production, where quality assurance is critical. The table below serves as a practical overview to support your evaluation of inspection technologies.
| Manual Visual Inspection (MVI) | Automated Visual Inspection (AVI) | |
|---|---|---|
| Description | Trained staff visually inspect each product for defects such as particles, cracks, or discoloration. Typically used for small batches or fallback control. | High-speed cameras and AI-assisted image processing systems automatically inspect products inline for defects with consistent accuracy. |
| Typically used | Small-scale production, special lots, fallback control | Mass production, pharmaceutical, medtech and food packaging lines |
| Advantages |
+ Flexible and easy to deploy + Low upfront costs + Allows human judgement for ambiguous cases |
+ High-speed inspection + Consistent, objective results + Full traceability (image archive, audit logs) + Scalable and future-proof + Easy inspection of variable data and comparison with system data (e.g. ERP, MES) |
| Disadvantages |
– Human fatigue & inconsistency – Subjective decisions – Labour-intensive & costly at scale – Limited documentation – Typically can’t assess non-Latin scripts (e.g. Arabic, Chinese) – Skilled inspectors are hard to find & retain |
– High initial investment – Complex validation & integration – Requires technical expertise & maintenance |
Choosing the right inspection method depends on your production environment, regulatory requirements, and quality expectations. Whether you’re looking to upgrade from manual inspection or optimise an existing AVI setup – our experts can help you evaluate the best solution for your needs.
Contact us to discuss your inspection requirements or to request a demo tailored to your production line.